Lean Manufacturing in the Pharmaceutical Industry: Reducing the Eight Wastes (Muda) for Enhanced Efficiency and Compliance
Discover how Lean manufacturing can help the pharmaceutical industry eliminate waste, boost efficiency, and maintain quality. Learn practical steps to streamline processes and ensure compliance.
Amin Nizar Thanawalla
10/29/20245 min read
Lean manufacturing, a production philosophy originating from the Toyota Production System, has gained traction across industries due to its effectiveness in reducing waste, improving efficiency, and maintaining quality. In the pharmaceutical industry, Lean manufacturing is especially relevant, as it addresses both operational and regulatory challenges. Applying Lean principles to identify and eliminate the eight wastes—often referred to as Muda—helps pharmaceutical companies streamline their processes, cut costs, and ensure compliance with stringent regulatory standards. This article explores the eight wastes within a pharmaceutical context and outlines practical strategies for reducing them.
Understanding the Eight Wastes (Muda) in Lean Manufacturing
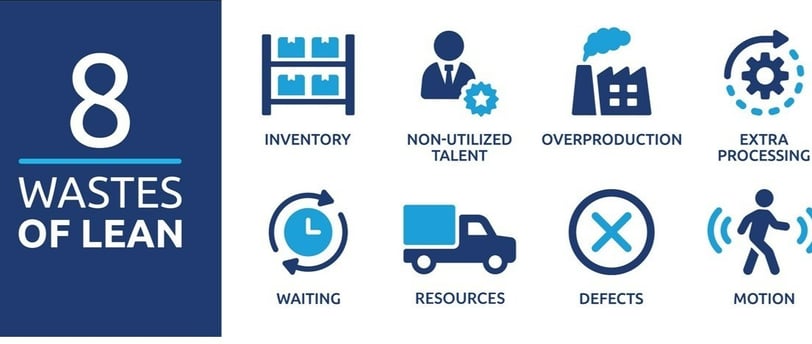
Lean defines eight types of waste, categorized as activities that do not add value to the product or service. Eliminating these wastes can improve quality, reduce lead times, and enhance patient safety in the pharmaceutical sector. Below, we delve into each waste and its implications for pharmaceutical manufacturing.
1. Transportation Waste
Definition: Waste from unnecessary movement of materials, products, or equipment within a facility.
Pharma Impact: Excessive transportation not only wastes time but also increases the risk of contamination, especially in cleanroom environments.
Lean Solution: Pharmaceutical companies can reduce transportation waste by optimizing plant layouts and implementing automated guided vehicles (AGVs) or conveyor systems to move materials. Efficient layout planning ensures that workstations, equipment, and storage areas are positioned to minimize movement, keeping products safe and reducing handling times.
2. Inventory Waste
Definition: Excess inventory that is not immediately required in the production process.
Pharma Impact: Excess inventory, including raw materials, intermediates, or finished products, ties up capital, requires additional storage, and risks expiry or obsolescence. For pharmaceuticals, holding too much inventory can lead to compliance issues and increased wastage.
Lean Solution: Adopting Just-in-Time (JIT) principles ensures that materials are ordered and received only when needed, reducing storage costs and the risk of expiration. Inventory management systems that track demand in real time can help align production closely with actual sales, reducing waste.
3. Motion Waste
Definition: Unnecessary movement of people, equipment, or machinery that does not add value.
Pharma Impact: In pharmaceutical manufacturing, excessive motion can slow down production and increase the risk of errors, particularly in cleanrooms where strict hygiene standards must be maintained.
Lean Solution: The 5S methodology—Sort, Set in Order, Shine, Standardize, and Sustain—helps reduce motion waste by ensuring tools and materials are easily accessible and organized. Lean also encourages ergonomic designs in workstations to minimize fatigue and reduce the potential for errors.
4. Waiting Waste
Definition: Time spent waiting for materials, equipment, or approvals that delay production.
Pharma Impact: Delays in production lead to extended lead times and wasted capacity, often causing bottlenecks that impact product availability. In regulated industries like pharmaceuticals, waiting for lab results or quality inspections can contribute significantly to this waste.
Lean Solution: Implementing Total Productive Maintenance (TPM) helps minimize equipment breakdowns, while scheduling tools and improved communication channels ensure that materials and resources are available as needed. Automation of quality control (QC) processes can also reduce waiting times by providing faster, more consistent results.
5. Overproduction Waste
Definition: Producing more than is needed, resulting in excess inventory and additional costs.
Pharma Impact: Overproduction in pharmaceuticals often leads to excess finished goods that may expire before use, resulting in costly waste.
Lean Solution: Lean encourages demand-driven production models like JIT, where products are made to meet actual demand. By aligning production with demand forecasts and implementing batch-sizing techniques, companies can reduce overproduction and minimize the risk of expired inventory.
6. Overprocessing Waste
Definition: Performing extra steps or using more resources than necessary.
Pharma Impact: In pharmaceuticals, overprocessing can manifest as redundant tests, excessive documentation, or complex protocols that don’t necessarily add value but increase time and cost.
Lean Solution: Streamlining processes to include only necessary steps, or "value-added" activities, helps minimize overprocessing waste. Standard Operating Procedures (SOPs) should be reviewed regularly to identify redundant steps, and automation can be introduced to minimize repetitive manual tasks, reducing errors and ensuring consistency.
7. Defects Waste
Definition: Production errors that lead to rework, waste, or product rejection.
Pharma Impact: Defects in pharmaceutical products can have serious consequences, including regulatory non-compliance, costly recalls, and potential harm to patients.
Lean Solution: Lean emphasizes quality at the source through mistake-proofing (Poka-Yoke) and root cause analysis. Implementing quality control measures at every stage of production reduces the chance of defects and helps maintain compliance with Good Manufacturing Practices (GMP). Continuous monitoring and corrective actions further help ensure quality consistency.
8. Underutilized Talent
Definition: Failing to leverage employees' skills, knowledge, or ideas for improvement.
Pharma Impact: In many pharmaceutical companies, strict protocols limit employees’ involvement in decision-making or innovation, leaving valuable insights unutilized.
Lean Solution: Lean encourages employee engagement by fostering a culture of continuous improvement, or Kaizen. Pharmaceutical companies can leverage cross-functional teams to brainstorm and implement process improvements, ensuring that staff members are empowered to contribute their expertise.
Lean Tools for Reducing Waste in Pharmaceuticals
Implementing Lean in pharmaceuticals requires a toolkit to address the industry’s unique challenges. Here are some essential Lean tools and methods that can help reduce the eight wastes:
Value Stream Mapping (VSM): Visualizing each step in a process to identify and eliminate non-value-added activities.
5S System: Organizes workplaces to reduce motion, waiting, and defect wastes while enhancing compliance with GMP.
Kanban and Just-in-Time (JIT): Pull-based systems ensure production is aligned with demand, helping prevent overproduction and excess inventory.
Total Productive Maintenance (TPM): Prevents equipment downtime and reduces waiting waste by ensuring machines are operational and available as needed.
Root Cause Analysis: Tools like the 5 Whys and Fishbone Diagram help identify the underlying causes of defects, allowing for effective corrective actions.
Standardized Work: Consistent processes reduce variation and errors, ensuring quality while improving efficiency.
Employee Involvement Programs: Kaizen encourages small, continuous improvements by engaging employees at all levels in identifying waste and suggesting improvements.
Benefits of Lean in the Pharmaceutical Industry
Adopting Lean manufacturing principles yields significant advantages for pharmaceutical companies, especially in highly regulated and competitive environments:
Enhanced Compliance: Lean’s emphasis on standardization and mistake-proofing helps companies meet GMP and regulatory requirements, reducing the risk of costly non-compliance.
Improved Quality: By addressing defects and overprocessing, Lean helps maintain high product quality, critical to patient safety and brand reputation.
Cost Reduction: Waste elimination leads to cost savings, particularly in inventory, rework, and processing, making Lean a valuable tool for optimizing operational budgets.
Increased Efficiency: Lean reduces lead times and improves resource utilization, allowing companies to respond more effectively to market demands.
Engaged Workforce: Lean’s focus on involving employees in continuous improvement fosters a culture of engagement, accountability, and morale, which is beneficial for productivity and innovation.
Greater Flexibility: Streamlined processes make it easier for companies to adapt to changes in demand or regulatory requirements, helping them remain competitive.
Challenges of Implementing Lean in Pharmaceuticals
While the benefits of Lean are substantial, implementing Lean in the pharmaceutical industry presents some unique challenges:
Regulatory Constraints: Changes to processes often require validation and approval, making Lean implementation slower than in other industries.
Risk of Resistance to Change: Employees used to traditional methods may resist Lean initiatives, requiring careful change management.
Upfront Investment: Lean tools like automation and real-time tracking systems may involve initial investment, but the long-term benefits often outweigh the costs.
Balancing Standardization with Flexibility: Pharmaceutical processes are highly standardized, so adopting Lean requires careful integration to maintain compliance while optimizing processes.
Lean manufacturing offers a powerful framework for enhancing efficiency, quality, and compliance in the pharmaceutical industry. By systematically identifying and eliminating the eight wastes, pharmaceutical companies can create streamlined processes that reduce costs, improve product quality, and enhance customer satisfaction. Lean principles, when implemented thoughtfully, lead to a culture of continuous improvement, where every team member contributes to operational excellence. For pharmaceutical companies, embracing Lean is not just about operational efficiency but also about meeting the highest standards of quality and safety for the benefit of patients.
